Binding Energy
Technicalities in a technical test
A discussion of binding energies in ordinary matter, exotic matter and potential implications.
A technical test will be attempted within the stream and that will be to launch a mid-stream zoom session where people can join with video / audio for live discussion whist simultaneously casting to Youtube.
Splitting an O-H bond
The energy required to split the O-H bond in water is 5.15eV
On 13 December 2014 I suggested here that
“Partially reduced Fe2O3 or similar H2 splitting catalyst in/on standard nickel helps to create P+ (H+) in the lattice. Fe3+ Al3+ helps to capture electrons”
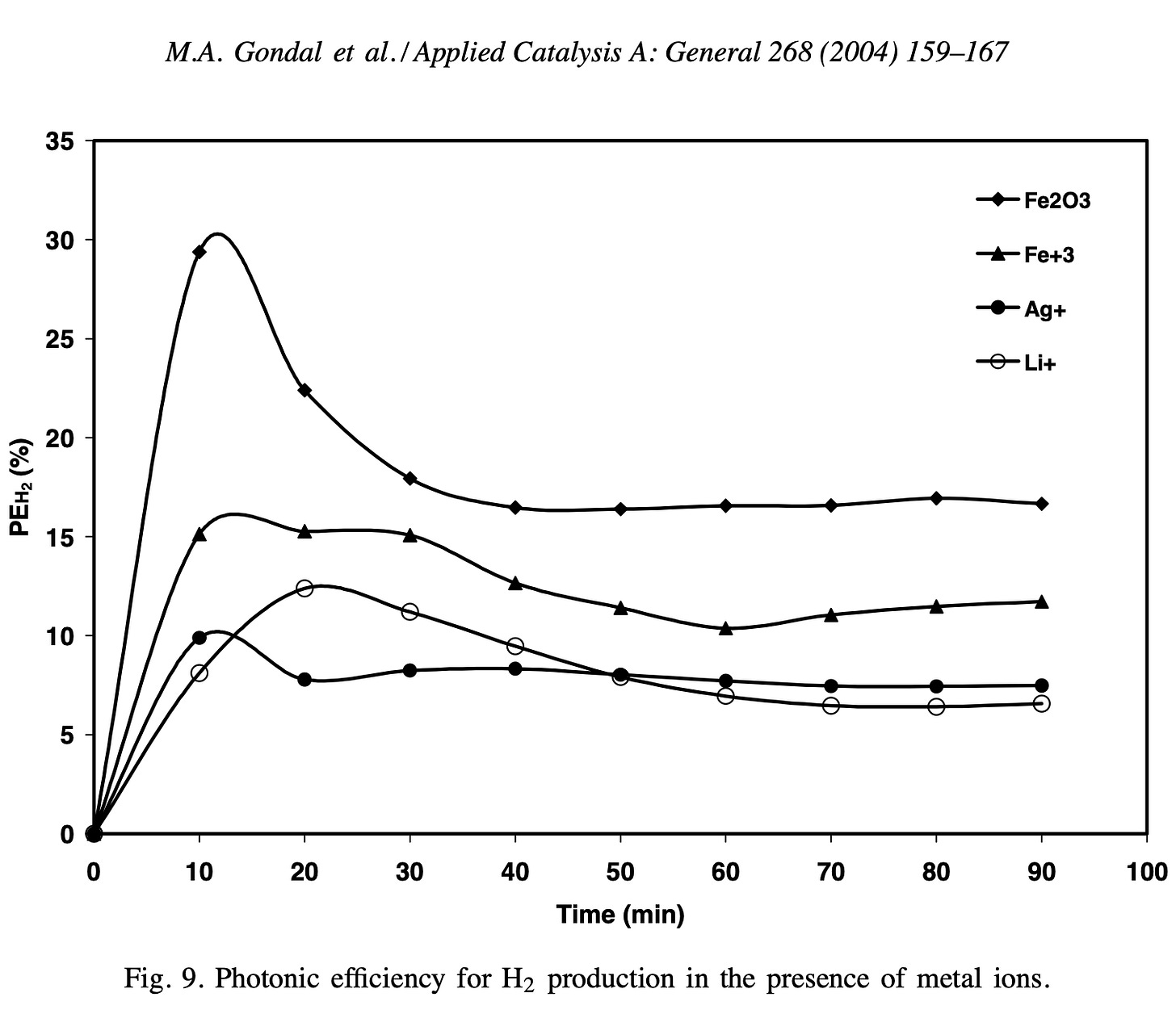
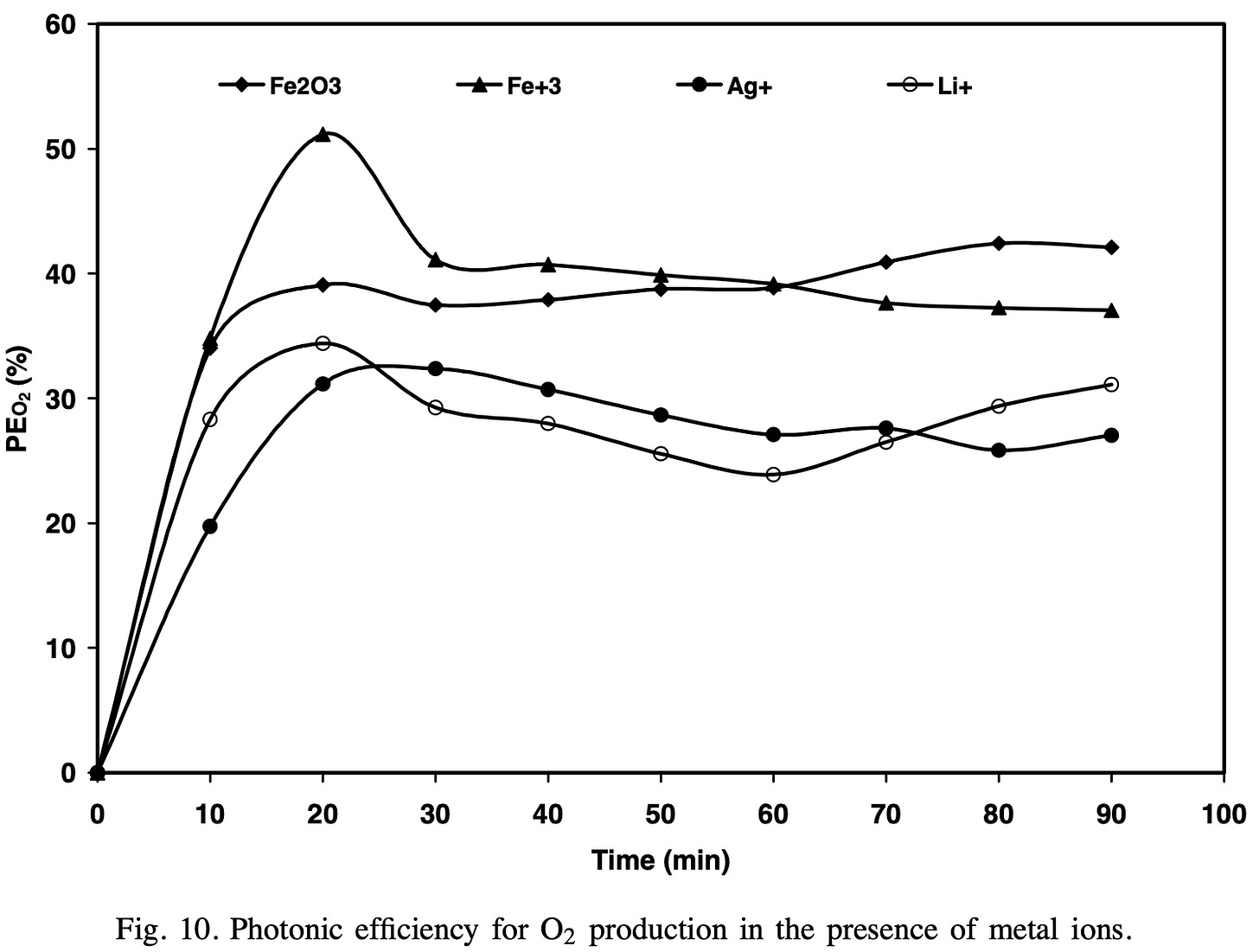
I based this view on this 2004 paper [1]. Which had the following two graphs.
In the paper, they make the following conclusions:
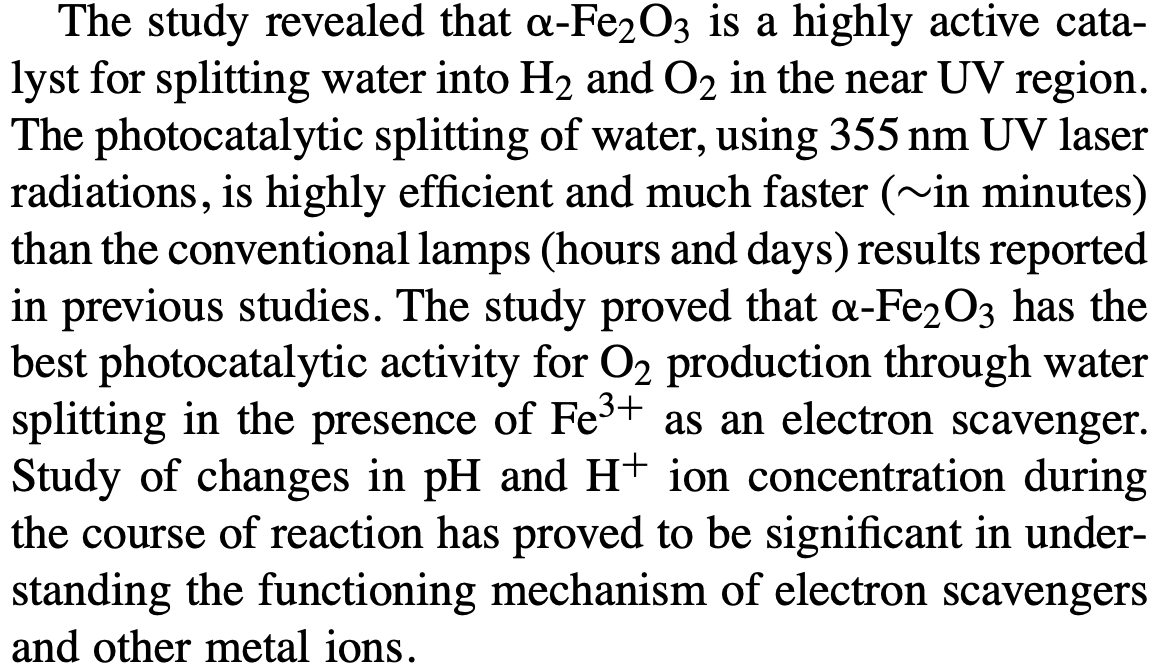
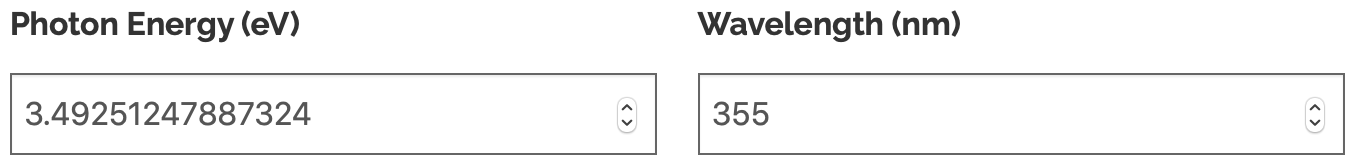
As is clear from above, they used a 355nm laser (here are some commercial products at this photon wavelength) by using this calculator we can see that the energy of a 355nm laser is a little over 3.49eV.
This 3.49eV is clearly below the 5.15eV O-H bond binding energy and so it is clear any catalytic process is stimulated by the impinging UV light.
More specifically, with reference to this phrase “The study proved that Fe2O3 has the best photocatalytic activity for O2 production through water splitting in the presence of Fe3+ as an electron scavenger.”
This is interesting for two reasons:
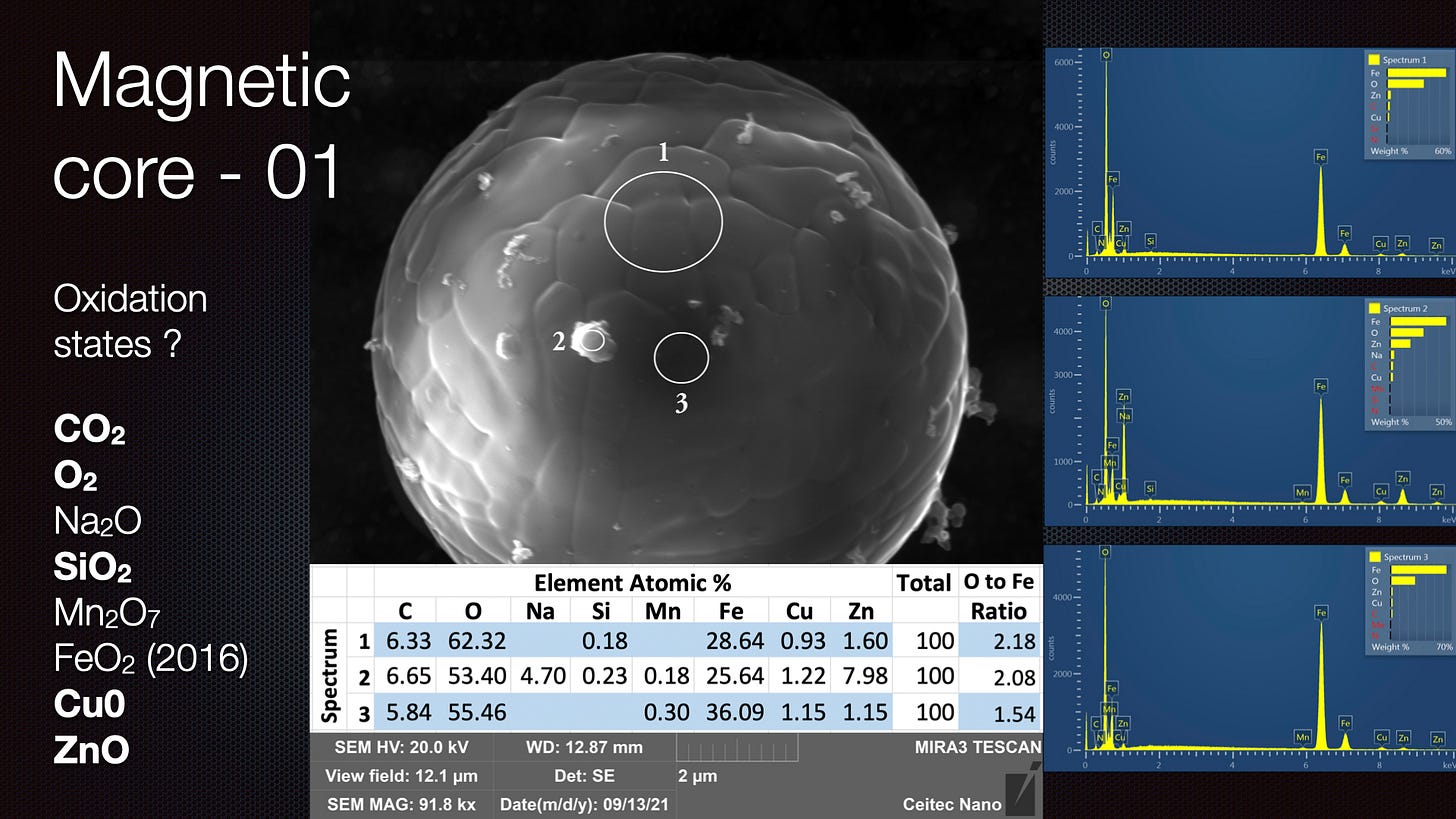
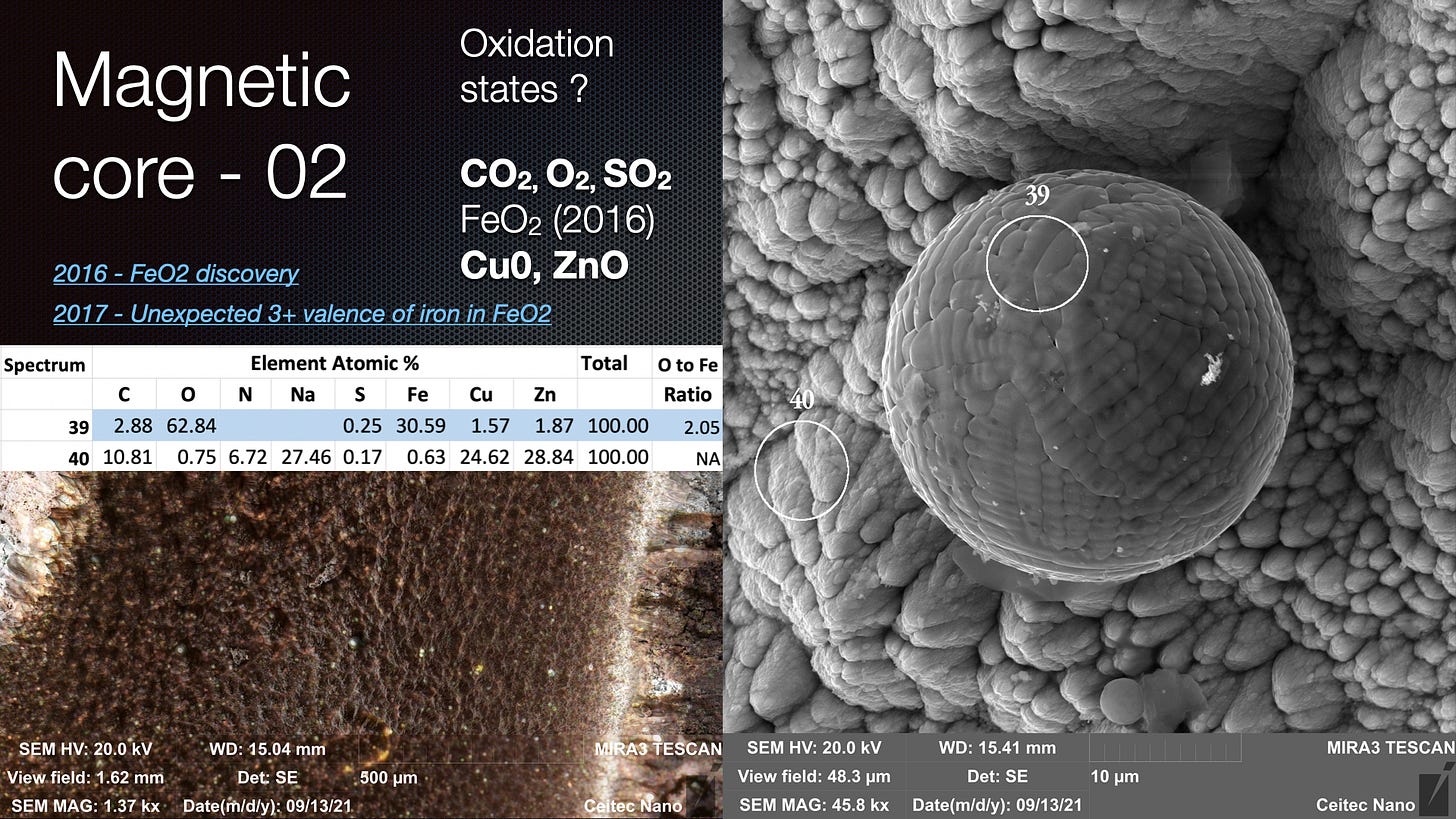
The paper I have referred to before on FeO2 is called “Unexpected 3+ valence of iron in FeO2, a geologically important material lying “in between” oxides and peroxides”
As I said, from first glance, it actually looked to me like the ‘FeO2’ crenelated spheres found in VEGA Valley were grabbing electrons from the Scanning Electron Microscope beam, leaving the surrounding area depleted and an ‘atmosphere’ around the ball itself.
I intuited that this could be leveraged for water splitting purposes and with subsequent reference to the 2004 and 2017 papers, I believe that could actually be the case. It appears that the 2017 paper determined Fe is in a 3+ state in FeO2 and Fe3+ has already been shown to catalyse water splitting in the 2004 paper. See my key images that I discussed again in the above and previous presentations.

If these spheres could be produced in bulk, then they might be able to be used in making highly efficient water splitting devices that could work merely by exposure to the sun allowing for passive production of HHO which can be piped and stored providing instant on-demand electrical via hydrogen fuel cells (H2 could be fractioned off) or instant on thermal production by burning of the HHO with a carbon containing additive.
Production of spheres via ULTR science
In the case of ULTR - you can see on the first and second crenelated spheres generated and analysed by Alan Golwater here:
that, when excluding other atoms, the Fe:O ratio is above 2 - however, there is so much carbon, and this can have a C:O ratio of 2 also and we also do not know if there are compounds containing C,O and H as we cannot see H on EDS. Moreover, the sample is on a carbon tape so it may have acquired C from that. So more testing needs to be done. The use of Cu mounting will help determine if these spheres have a ratio above 2. As I said, in the presentation at the top of this blog, in the ULTR experiments - the action is occurring in a water rich environment - VEGA is a low pressure residual air. There is less chance for contamination in the VEGA experiments if the spheres are suspended in a micro Ball Lightning / cohering structure during formation. The VEGA Valley sphere surfaces certainly look a lot 'purer'.
In the presentation above, I noted that if these things could be produced via ULTR - it would be far easier to make them in bulk and we already know we can collect them via magnets. If they are FeO2 (or largely) or meet or surpass the oxidation level claimed by the Japanese inventor [3] to cause hydrogen synthesis, then we are on to a winner! From a translation of the Japanese patent application
“When NaOH or KOH is heated above its melting point, nano-order fine particles scatter from its liquid surface into an oxygen-free atmosphere in the air, capturing the supplied water vapor molecules and creating a stainless elemental atmosphere (Cr-Ni). -Fe) reacts to form higher order alkali metal-transition metal oxide films such as Na3Fe5O9, K3Fe5O9, NaxFeyCrzOw (x, y, z, w are integers), which are conductive, hydrophilic and it has high hardness, magnetism, heat resistance, does not melt even at 1000°C, has high hydrogen storage capacity, has the ability to separate hydrogen into hydrogen ions (H+) and electrons (e-), and is an electrode for fuel cells. It can be used as a (negative electrode), and further increases the possibility of a nuclear reaction occurring when the alkali metal-transition metal oxide is formed on the stainless steel surface.”
NOTE: KOH melts at 360°C and Holmlid is passing his D2 through iron oxide dehydrogenation catalyst with potassium salts heated to around 400°C.
In the case of production, the foil needs to be static for the coherence to build up at the node long enough for the spheres to form. I think that it would be better to have vertical layers in the water column - I think that the ‘figure 8s’ are self-centering - and it would be like that video I shared of fluid droplets suspended in the air which I noted were IN PAIR - just like the 8s in ULTR experiments in water.

We know from various ULTR tests that the vortex can form in the water column so I suggest a 3D printed stackable mesh with the foil loosely held in each ‘floor’ of the stack - like a layer cake. The foil would be loose enough so that the parts could move to the requisite place in the water column where the standing wave nodes can perform their synthesis function.
Direct electric potential from ambient heat and water?
If it is able to passively split water via ambient heat, which is in line with the claim made to me privately by Francesco Celani about body temperature splitting of water with his wires, then it may be possible for it to directly produce an electric potential in the process.
References
GONDAL, M., HAMEED, A., YAMANI, Z., & SUWAIYAN, A. (2004). Production of hydrogen and oxygen by water splitting using laser induced photo-catalysis over FeO. Applied Catalysis A: General, 268(1-2), 159–167. doi:10.1016/j.apcata.2004.03.030
Streltsov, S. S., Shorikov, A. O., Skornyakov, S. L., Poteryaev, A. I., & Khomskii, D. I. (2017). Unexpected 3+ valence of iron in FeO2, a geologically important material lying “in between” oxides and peroxides. Scientific Reports, 7(1).
doi:10.1038/s41598-017-13312-4Comment originally sent to me by Triton Jäger on the video "VEGA - Further look at VEGA Valley SEM/EDS data" about the Japanese researcher developing higher oxidation states of Fe and assessing their properties.
"Hello Bob, well done again for your tremendous enthusiasm; thanks for that !! so, concerning your higher oxides of iron, you should definitely consult the work of the Japanese ISHIKAWA YASUO; this researcher manages to transmute, in large mass, many gases into hydrogen, using this type of super-oxygenated catalyst which is created spontaneously during the reaction: and this only by chemical means: he has filed a lot of patents, often in Japanese: here the patent links speaking more specifically of this super iron oxide and derivatives ; the first is in Japanese, the second a translation from Google Translate:"



Here is the comment originally sent to me by Triton Jäger on the video "VEGA - Further look at VEGA Valley SEM/EDS data" about the Japanese researcher developing higher oxidation states of Fe and assessing their properties.
"Hello Bob, well done again for your tremendous enthusiasm; thanks for that !! so, concerning your higher oxides of iron, you should definitely consult the work of the Japanese ISHIKAWA YASUO; this researcher manages to transmute, in large mass, many gases into hydrogen, using this type of super-oxygenated catalyst which is created spontaneously during the reaction: and this only by chemical means: he has filed a lot of patents, often in Japanese: here the patent links speaking more specifically of this super iron oxide and derivatives ; the first is in Japanese, the second a translation from Google Translate:"
https://drive.google.com/file/d/1Rjl-Pyo7ZQBrUZS6As3hc0zYxak_b-pZ/view?usp=sharing
https://docs.google.com/document/d/11Ip_1f_1bUhrHAAm2c4yjEuLFZDR1jgQ/edit?usp=sharing&ouid=102978756288560332775&rtpof=true&sd=true
On 13 December 2014 I suggested here:
https://www.facebook.com/MartinFleischmannMemorialProject/photos/a.587293604634676/883520658345301
"Partially reduced Fe2O3 or similar H2 splitting catalyst in/on standard nickel helps to create P+ in the lattice. Fe3+ Al3+ helps to capture electrons - see here:
Production of Hydrogen and Oxygen by Water Splitting Using Laser Induced Photo-Catalysis Over Fe2O3"
http://bit.ly/1yNwUY2
Wow, this article has grown very fast! I am still not finished watching the video and came here to comment about the Binding energy per nucleon chart that felt really familiar to me as I had seen it in the Cardone et al Mercury transmutation papers and talks, where he used it to make the point that their results pointed that the “Deformed Time Space” reactions could result in element synthesis completely outside the expected results going up or low in the chart, starting from Hg.
In particular in your video the mention of the BEN for Br being in an odd spot that would not ease the reaction for having it as product, called my attention, because by far, the element that Cardone et al found in higher concentration in the solids recovered from Mercury was Bromine (albeit they warn this could be an artifact of the separation and sampling process, as the solid was really hard to analyze from a quantitative point of view, being so heterogeneous in nature). I recall a while ago you made the Parkhomov’s table query for the elements on Cardone’s Mercury experiment (mainly Hg and Al plus the atmospheric gases) and Bromine was a possible product, so the end products will always depend on the starting ones and the duration of the experiment as products become reactants as time progresses.